By CCHR International
July 28, 2015
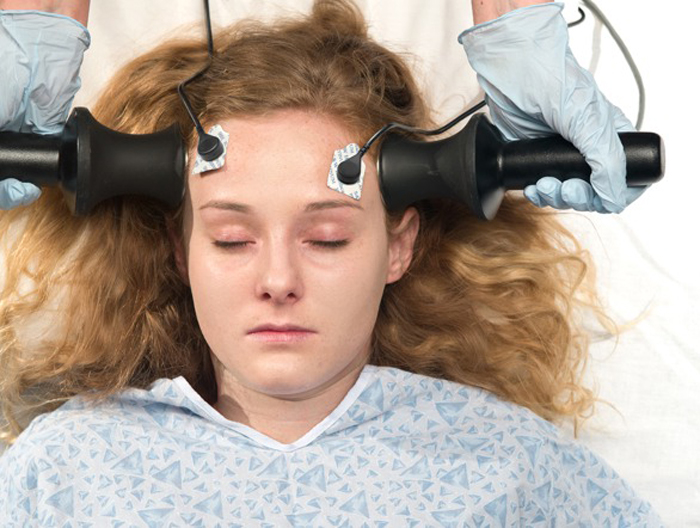
Citizens Commission on Human Rights has written to Abiy B. Desta, Ombudsman over the neurological devices section of the Food and Drug Administration (FDA) demanding he investigate the FDA’s more than five-year procrastination regarding a decision confirming the dangers of electroshock treatment (ECT) devices. More than 100,000 Americans are still electroshocked every year, suffering the force of 460 volts of electricity searing through their brain, causing brain damage and severe memory loss. This includes adolescents.
Jan Eastgate, President of CCHR International wrote to Desta alerting him to her January 2011 testimony before the FDA’s Neurological Devices Advisory Panel investigating the risk of classification of the ECT device. The FDA was reviewing whether the ECT device should remain under the FDA’s highest risk category of class III or be reduced to class II. Were the latter approved, it would enable psychiatrists to shock and damage thousands more, including children, and increase their profits in an already $1.2 billion a year ECT industry.
Because the device was in use prior to a 1976 amendment regarding medical devices, it continued to be used without the usual FDA required Pre-Marketing Approval (PMA) that provide clinical trial evidence of safety and efficacy.[i] Essentially, since 1976, more than 4 million Americans and since 2011 some 500,000, have been guinea-pigs to psychiatrists’ shock box.
In November 2011, the then FDA Assistant Commissioner for Legislation Jeanne Ireland assured Senator Charles Grassley that the FDA would report on the ECT device by December 31, 2012. Twenty-seven months later, there’s yet to be a decision, despite the FDA Advisory panel agreeing that the device was sufficiently dangerous that it remain in class III and that more than 80% of respondents to the FDA’s ECT Executive Summary in 2011 wanted stricter oversight and even a ban on electroshock.[ii]
Eastgate says that the FDA has missed every deadline set by Congress and every timetable it set for itself to deal with the ECT device. It not only ignored Congressional mandates in 1990 and 2009 to comply with the 1976 Safe Medical Devices Act to get manufacturers of the ECT device to submit PMAs, and missed the December 2012 deadline, it has consistently collaborated with the American Psychiatric Association (APA) to rely upon “selective,” biased research rather than insist upon clinical trials.
Since 1978, the APA’s ECT Task Force reports have been prepared by committees whose members include psychiatrists with conflicts of interest with ECT device makers:
- Richard Weiner, a psychiatrist who designed shock devices for Mecta and later worked for Somatics—both ECT device manufacturers—chaired the 1986-1990 APA Task Force.[iii]
- In 1982, the APA filed a petition to have the device classified as Class II based on their own selective review of literature—not clinical trials proving safety. Psychiatrist Max Fink, who narrates ECT videos for Somatics, from which he receives royalties, wrote the APA’s first ECT Task Force report upon which the APA petition was largely based.
Electro-shocking Children
Eastgate told Desta: “The lack of monitoring of ECT usage, its adverse effects, including accurate reporting of deaths, is a national scandal. That it is still administered to children and adolescents, despite the device being in a Class III device and without a PMA, is unconscionable.”
- She pointed to the Western Australian Mental Health Act last year banning the use of ECT on those younger than 14 years old. The law imposes a (AUS)$15,000 fine on anyone performing the procedure on a child under 14.[iv]
- In Sicily, Italy, ECT is banned for all ages, which Eastgate says should be done globally.
In concluding her complaint to Desta, Eastgate asked what the Ombudsman’s Office will do to ensure that there is full FDA transparency and a final determination regarding ECT that is not based on biased industry studies steeped in conflicts of interest. According to Eastgate, “When someone receives high voltage shock, they’re told to call 911. But psychiatrists expect you to subject your brain to this. This is about as therapeutic as the electric chair and its time the FDA protected citizens from this. If there are no PMAs, the ECT device must be removed from the market.”
—
References:
[i] “FDA lax on scrutiny of some medical devices, report finds,” USA Today, 15 Jan, 2009, http://usatoday30.usatoday.com/news/health/2009-01-15-medical-devices_N.htm.
[ii] http://www.cchr.org/ect-fda-advisory-panel-electroshock-device.html.
[iii] Linda Andre, Doctors of Deception: What They Don’t Want You to Know about Shock Treatment (Rutgers University Press, 2009), p. 144.
[iv] http://www.abc.net.au/news/2014-10-17/mental-health-bill-passes-wa-parliament/5822874.


SHARE YOUR STORY/COMMENT