 The documented risks of these drugs are provided so the public can make informed, educated decisions. Metadate is a stimulant drug, classified by the U.S. Drug Enforcement Administration (DEA) as Schedule II, in the same class of highly addictive drugs as morphine, opium and cocaine. The DEA states that the use of stimulants can lead to “severe psychological or physical dependence” and that “these drugs are also considered dangerous.” Metadate is also known as Concerta, Concerta LP, Concerta Oros, Daytrana, Equasym, Equasym XL, Metadate CD, Metadate ER, Methylin, Methylin ER, Methylphenidate, Ritalin, Ritalin LA, Ritalin SR.
The documented risks of these drugs are provided so the public can make informed, educated decisions. Metadate is a stimulant drug, classified by the U.S. Drug Enforcement Administration (DEA) as Schedule II, in the same class of highly addictive drugs as morphine, opium and cocaine. The DEA states that the use of stimulants can lead to “severe psychological or physical dependence” and that “these drugs are also considered dangerous.” Metadate is also known as Concerta, Concerta LP, Concerta Oros, Daytrana, Equasym, Equasym XL, Metadate CD, Metadate ER, Methylin, Methylin ER, Methylphenidate, Ritalin, Ritalin LA, Ritalin SR.
To see the total figures from IQVia on the number of people taking stimulants in the U.S., click here.
Please note: No one should attempt to get off of psychiatric drugs without a doctor’s supervision. To help find medical practitioners in your area, click here.
Also, read the FDA’s Metadate Medication Guide for more information. MedGuides include “the particular serious and significant public health concern that has created the need for the Medication Guide” and notes “pediatric risks.” (Note: Unfortunately, the FDA MedGuides only work on a desktop, not on a mobile device. Please complain to the FDA to make their public advisories accessible to all. 1-888-INFO-FDA or 1-888-463-6332.)
Metadate Drug Warnings:
There have been 32 drug regulatory agency warnings from nine countries (Australia, Canada, Denmark, France, Japan, New Zealand, Singapore, United Kingdom and United States) on Metadate (or methylphenidate). These include the following (note that some warnings cite more than one side effect, so the list below may not be equal to the total number of warnings):
16 warnings on Metadate causing cardiovascular disorders
8 warnings on Metadate causing heart problems
5 warnings on Metadate causing death or sudden death
4 warnings on Metadate causing mania or psychosis
4 warnings on Metadate causing hallucinations
3 warnings on Metadate causing depression
3 warnings on Metadate causing heart attacks
3 warnings on Metadate causing skin conditions
3 warnings on Metadate causing addiction or dependence
2 warnings on Metadate causing eye problems
2 warnings on Metadate causing involuntary movements
2 warnings on Metadate causing stroke
2 warnings on Metadate causing suicide risk or attempts
2 warnings on Metadate causing agitation
2 warnings on Metadate causing seizures or convulsions
2 warnings on Metadate causing drug abuse
1 warning on Metadate causing hostility or aggression
1 warning on Metadate causing anxiety
1 warning on Metadate causing liver problems
1 warning on Metadate causing abnormal behavior
1 warning on Metadate causing sleep problems
1 warning on Metadate causing blood pressure changes
Metadate Drug Studies:
There have been 25 studies done in 14 countries (Australia, Belgium, Canada, China, Denmark, Germany, Hungary, Ireland, Italy, Netherlands, Scotland, South Korea, United Kingdom and United States) on Metadate (or methylphenidate). These include the following (note that some studies cite more than one side effect, so the list below may not be equal to the total number of studies):
6 studies on Metadate causing cardiovascular disorders
5 studies on Metadate causing lack of efficacy
4 studies on Metadate causing heart problems
2 studies on Metadate causing sleep problems
2 studies on Metadate causing blood pressure changes
2 studies on Metadate causing sudden death
2 studies on Metadate causing drug abuse
1 study on Metadate causing heart attacks
1 study on Metadate causing hallucinations
1 study on Metadate causing homicidal ideation
1 study on Metadate causing mania or psychosis
1 study on Metadate causing violence
1 study on Metadate causing stunted growth
1 study on Metadate causing abnormal behavior
1 study on Metadate causing cognitive impairment
1 study on Metadate causing involuntary movements
1 study on Metadate causing irritability
1 study on Metadate causing lowered bone mass
1 study on Metadate causing nervous system disorders
1 study on Metadate causing suicide risk or attempts
1 study on Metadate causing withdrawal reactions
Adverse Reaction Reports Filed with the US FDA: There have been 7,473 adverse reactions reported to the US FDA in connection with Metadate (methylphenidate).
The FDA estimates that less than 1% of all serious events are ever reported to it, so the actual number of side effects occurring are most certainly higher.
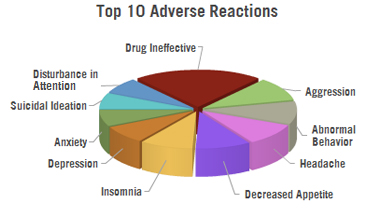
- 775 cases of the drug being ineffective
- 353 cases of aggression
- 343 cases of abnormal behavior
- 336 cases of headache
- 334 cases of decreased appetite
- 318 cases of insomnia
- 276 cases of depression
- 250 cases of anxiety
- 241 cases of suicidal ideation
- 236 cases of disturbance in attention
Documented Side Effects of Metadate (methylphenidate):
Source: Physicians Desk Reference, National Institutes of Health’s Medline Plus, and/or the drug label.
Agitation/hostility
Blood pressure changes
Changes in vision/ blurred vision
Depression
Hallucinations
Hives
Hypersensitivity
Insomnia
Involuntary tics/ twitching
Irregular heartbeat
Loss of appetite
Mood changes
Muscle tightness
Nausea
Nervousness
Numbness
Restlessness
Seizures
Sexual dysfunction
Slow/difficult speech
Stomach pain
Uncontrollable movement of a body part
Weight loss
Click here to learn more >>
This brochure is a simple guide that documents the dangerous and deadly side effects of the drugs prescribed to millions of men, women and children diagnosed with bogus mental disorders.


 Download The Side Effects of Common
Download The Side Effects of Common
SHARE YOUR STORY/COMMENT